Maximum quality and reliability
through prevention, control,
and mitigation of risks
the Minerva way
We create diagrams and illustrations of your process and systems to identify, assess, and prioritize risks. Using database analysis, and Minerva-led risk meetings, we minimize the time spent by your personnel in the risk reduction effort.
The resulting controls and mitigations are integrated into your products and software risk files for reduced quality issues and increased reliability meeting and exceeding ICH Q10 and ISO13485:1016.
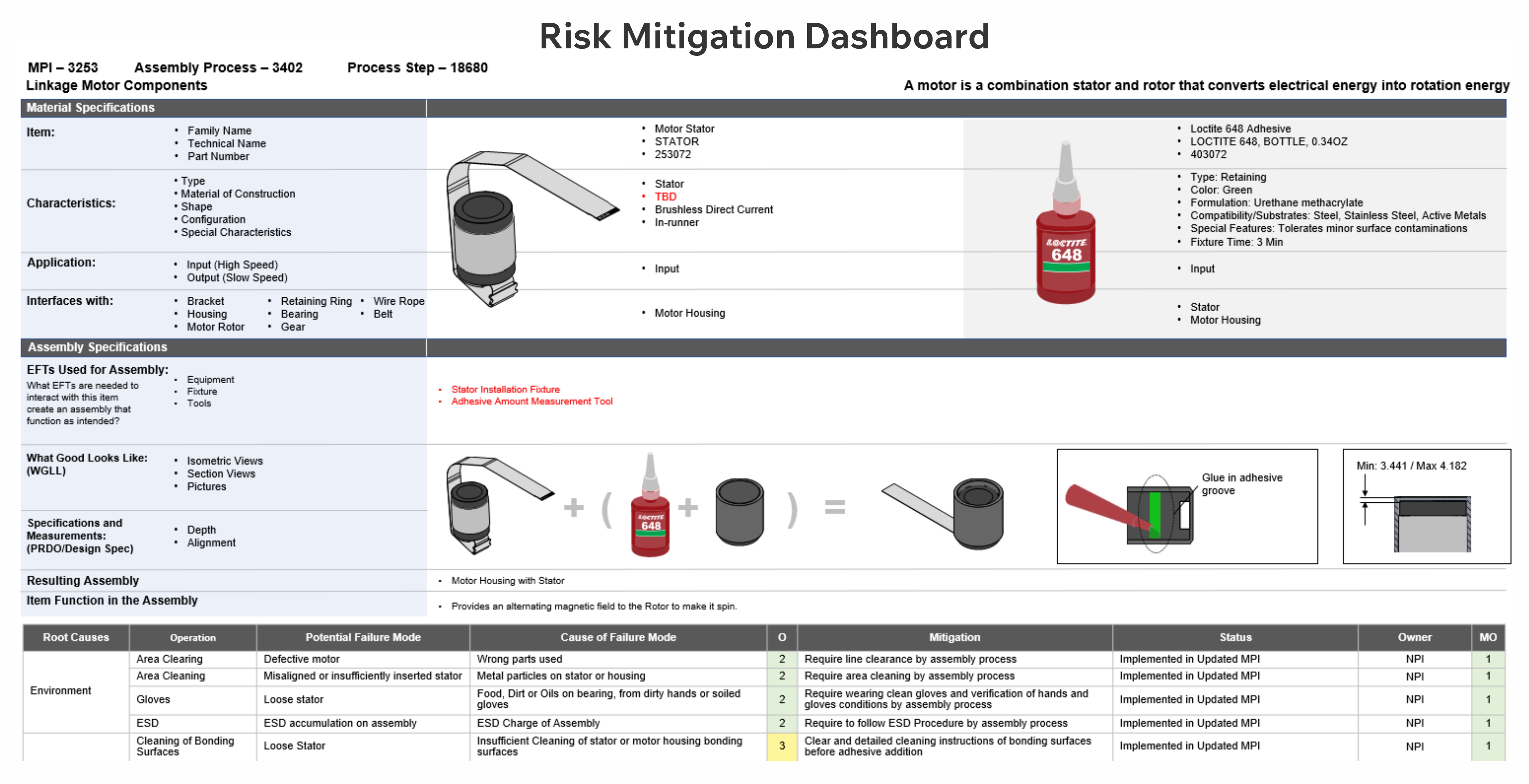
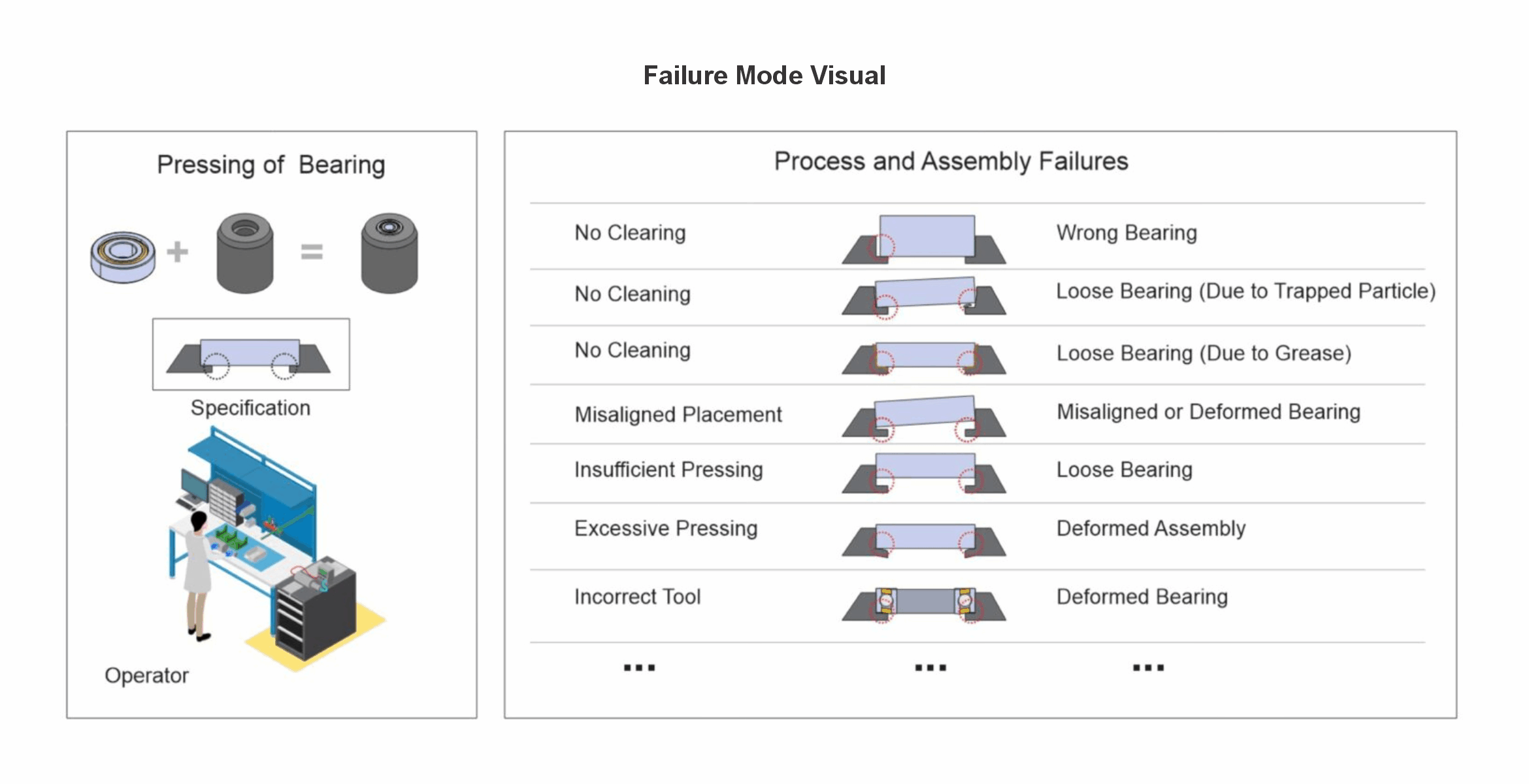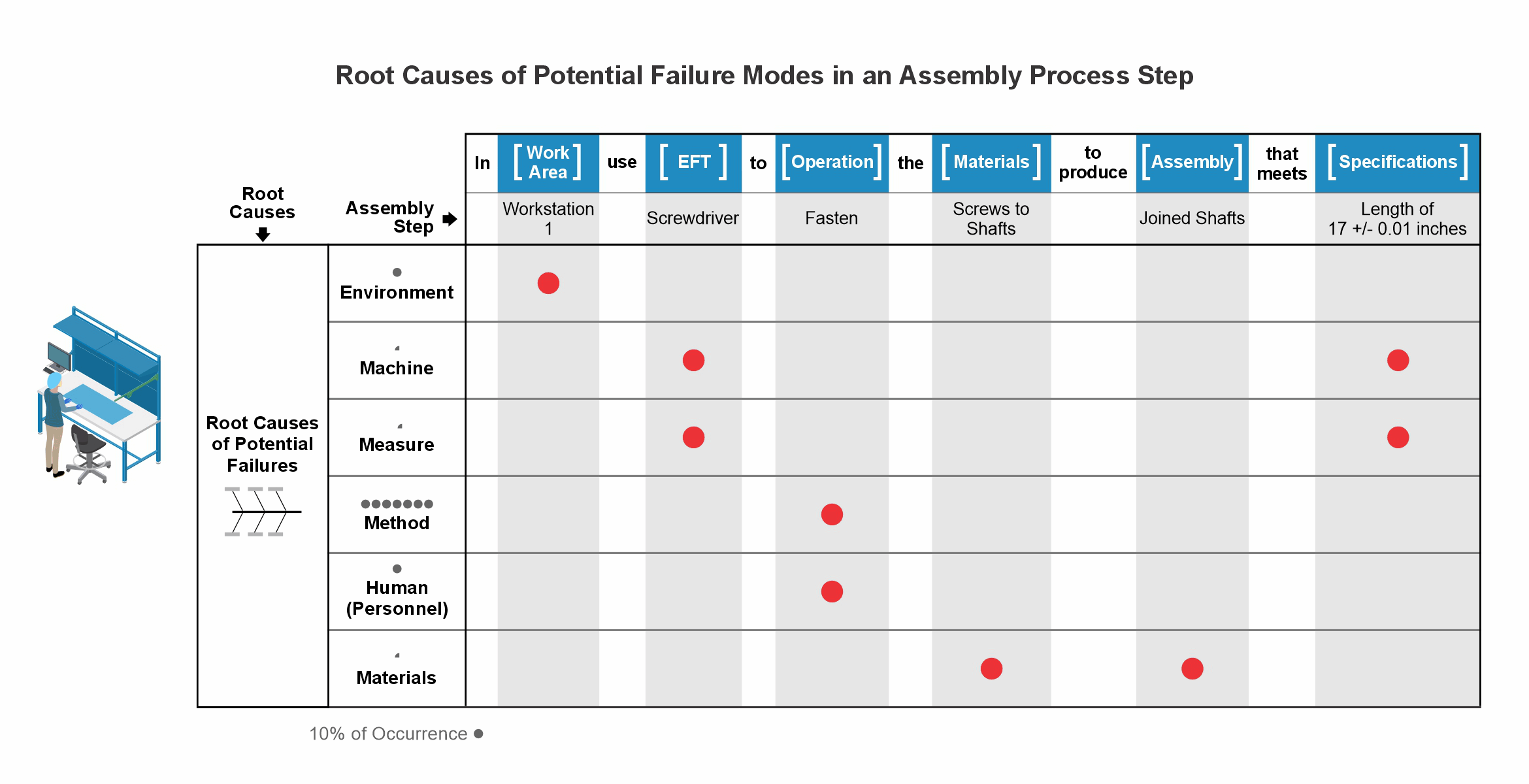
Our comprehensive risk methodology includes highly visual and digitalized root-cause analysis, risk assessment, and risk mitigation, resulting in design packages, operational and test procedures, and V&V protocols directly from the digital risk file.
Minerva provides complete risk management services including risk planning, assessment, control, mitigation, communication, monitoring and risk-benefit analysis for all your processes and systems, meeting and exceeding applicable CFR21 Part 820 Quality System Regulation, ICH Q9 Guidelines on Quality Risk Management, ISO 13485 Quality Management System and ISO 14971 Medical Device Risk Management, among others.
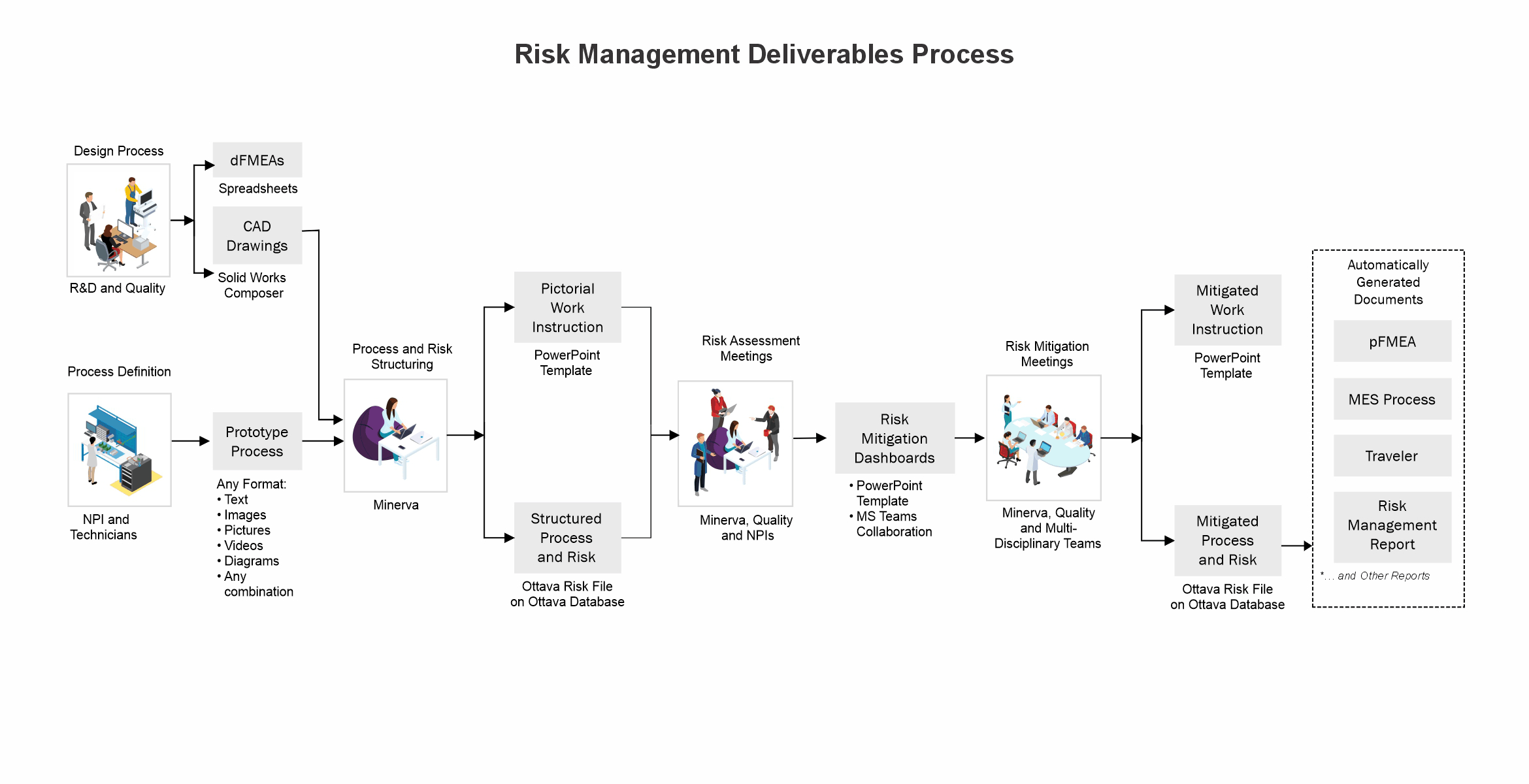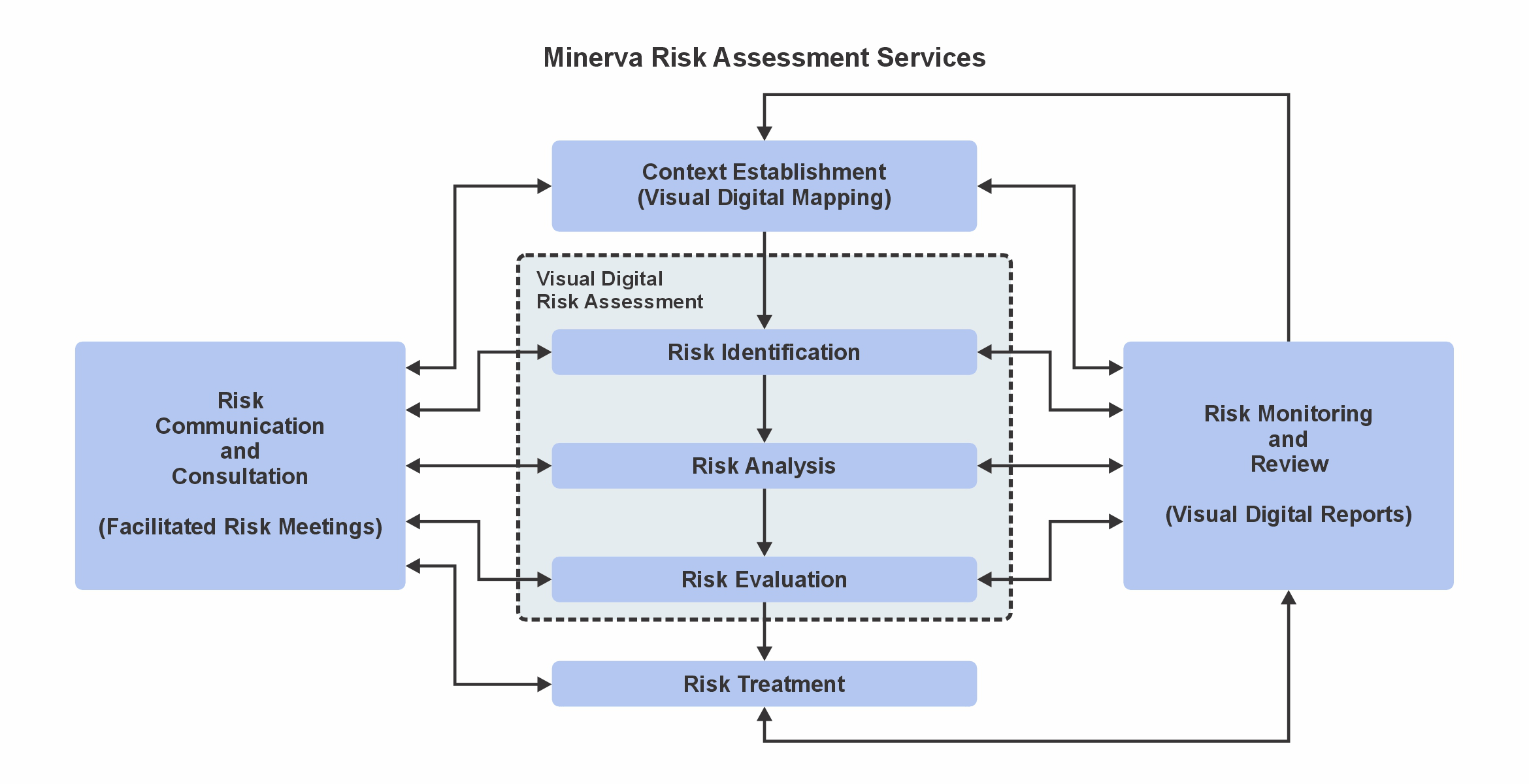
The integration of visuals in a digitalized risk management process is optimal for the identification of potential failures and the creation of controls resulting in minimum residual risks.
Minerva digitalizes manufacturing procedures and records and uses the experience of previous technical and quality issues to create highly visual risk files for risk mitigation meetings and failure prevention.
The Minerva supported risk assessment process helps your team in the development of controls to prevent failure modes, and the reduction of design, process, and use risks.
Minerva’s experienced risk meeting facilitators improve meeting efficiency, reducing the time taken from valuable resources during the risk management process.
Ensure verification and validation (V&V) activities are traceable to your design outputs and requirements with our integrated record creation and updates, during the design, prototype development, V&V, and commercialization phases.
Qualification protocols directly created from digital risk files in shared databases produce protocols and reports that are automatically traceable to related requirements, specifications, and tests.
the Minerva way