Product development support and investigation services for optimal productivity and quality
Align and standardize your development activities with Minerva’s integrated product development support. Minerva’s SME team with manufacturing, quality, visualization, and digitalization experience will facilitate low-risk, fast decision making for a fully compliant product development of your advanced therapies.

Benefit from our SMEs’ experience working with thousands of batches in manufacturing phases like apheresis collection, activation, transduction, expansion, formulation, and cryopreservation, and laboratory tests like cell count and viability, potency, and purity analysis, among others.
Improve understanding and communication of complex processes through visual models enabling alignment, better decision-making, collaboration, and risk reduction.
Convert your process information and experience into digital knowledge for optimal pharmaceutical quality risk management and sustainable GMP compliance consistent with ICH Q9.
Implement a highly visual, digital risk assessment framework to identify, evaluate and mitigate risks across all development phases, ensuring all decisions are risk based.
Combine our technical and quality investigation capabilities throughout all your development stages and operational areas to have continuous phase-appropriate response, resolution, and reduction of issues.
Learn more about Minerva’s C3 technical investigations and R3 quality investigations here.

Address issues and opportunities with combined technical and quality investigations throughout process development, GMP process definition, commercialization, and continuous improvement, provided by the Minerva multidisciplinary team.
Bridge your present process and your automation aspirations for advanced therapy medicinal products (ATMPs) with Minerva’s technical support. Minerva’s SME team with combined process, automation, and robotics experience will facilitate the transition from manual operations into practical, automated, and robot-assisted manufacturing and quality processes.
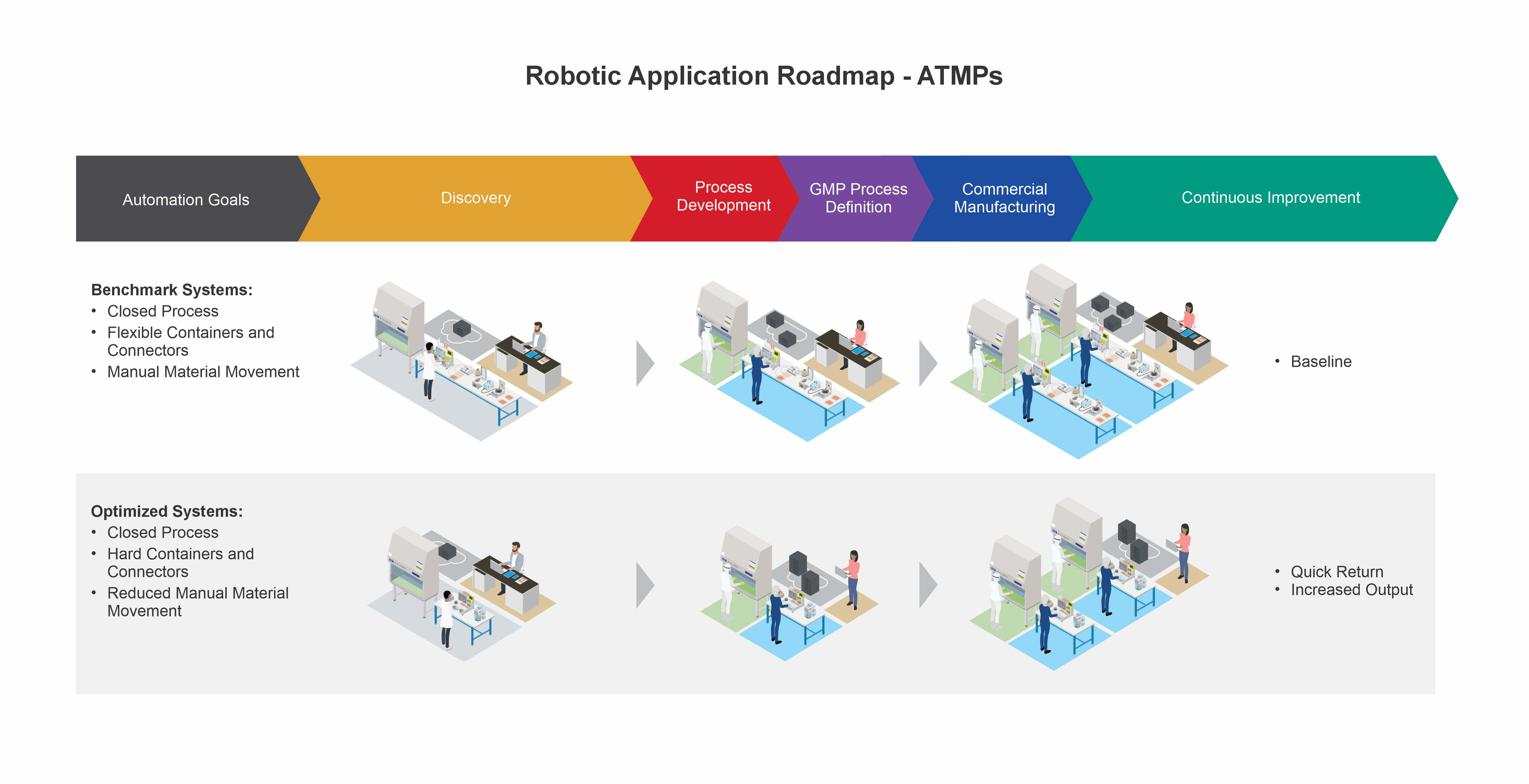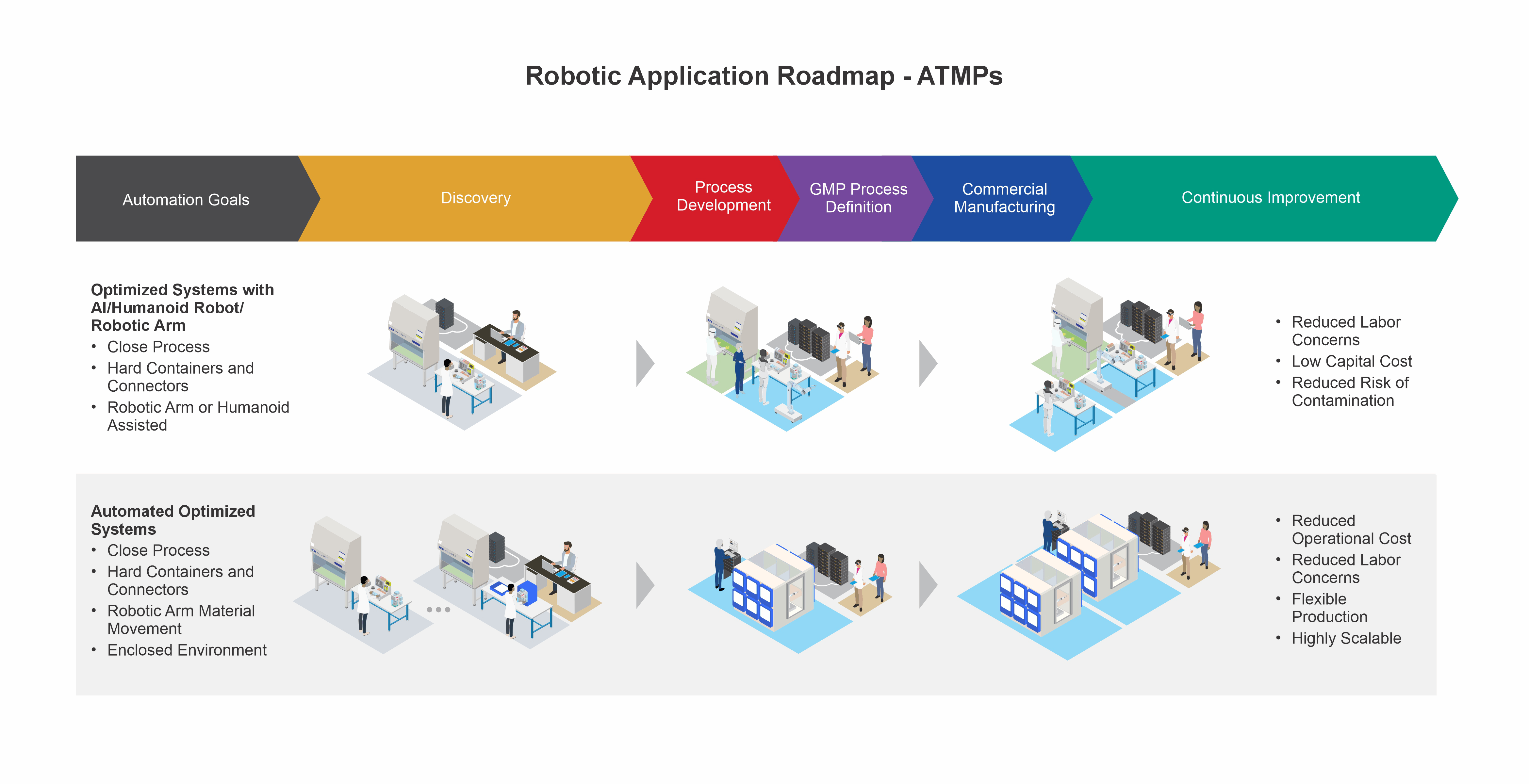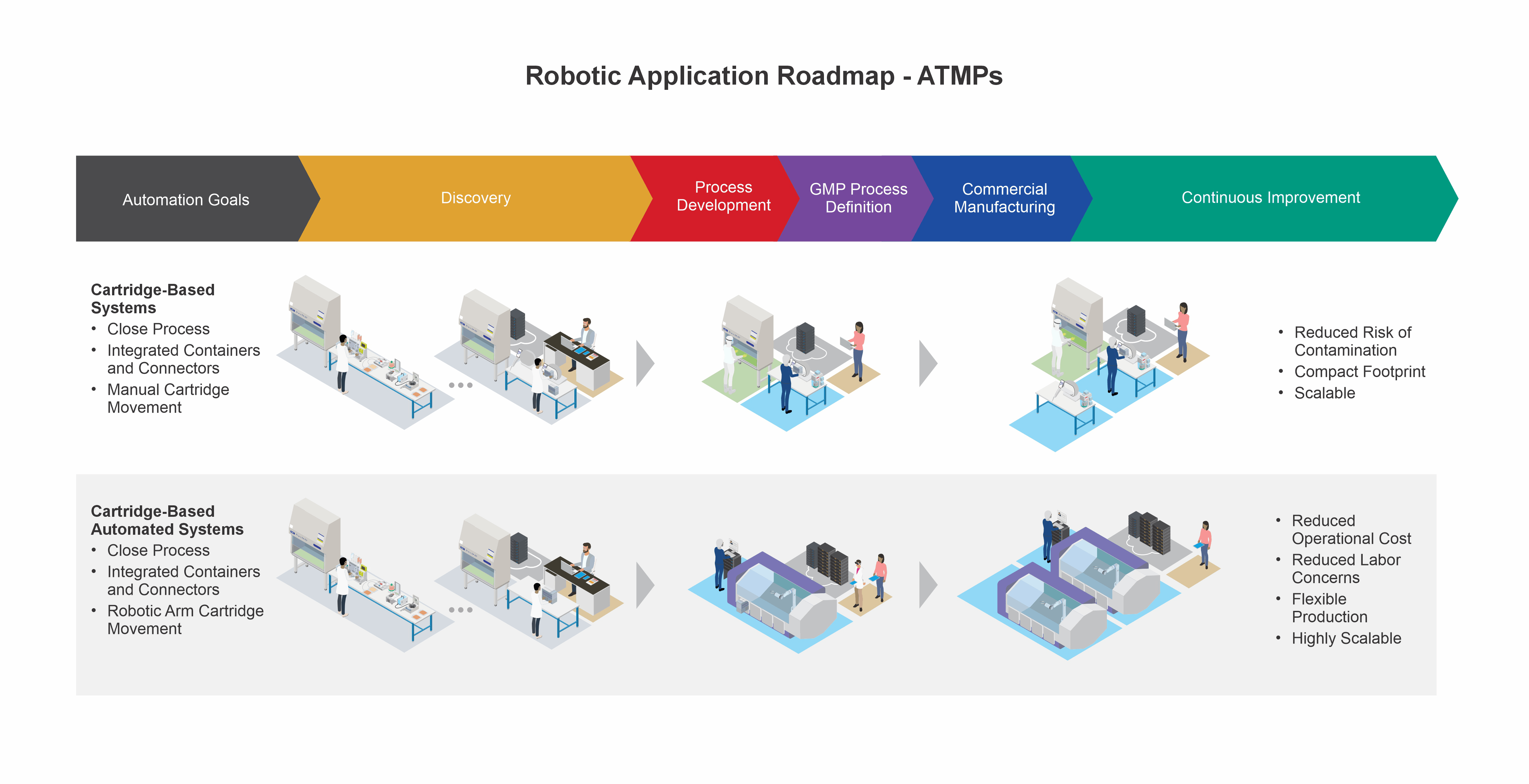
Achieve an automated process that fits your advanced therapy manufacturing needs with our SMEs that have combined experience in advanced therapies, automation modeling, and robotics implementation.
Capture and digitalize your combined scientific, manufacturing, and quality data the Minerva way to ensure the correct baseline and structure for automation is in place, for outcomes that are right the first time.
Advancing cell and gene therapy manufacturing and quality with innovative solutions and technologies.